News
2024
March
- Congrats to professor Li for being awarded for the 2024 Shanghai Science and Technology System “March 8th Red-Banner Pacesetter” !
- Cancer Systems Biology group contributes a research paper entitled “Applying machine learning for multi-individual Raman spectroscopic data to identify different stages of proliferating human hepatocytes” in iScience.
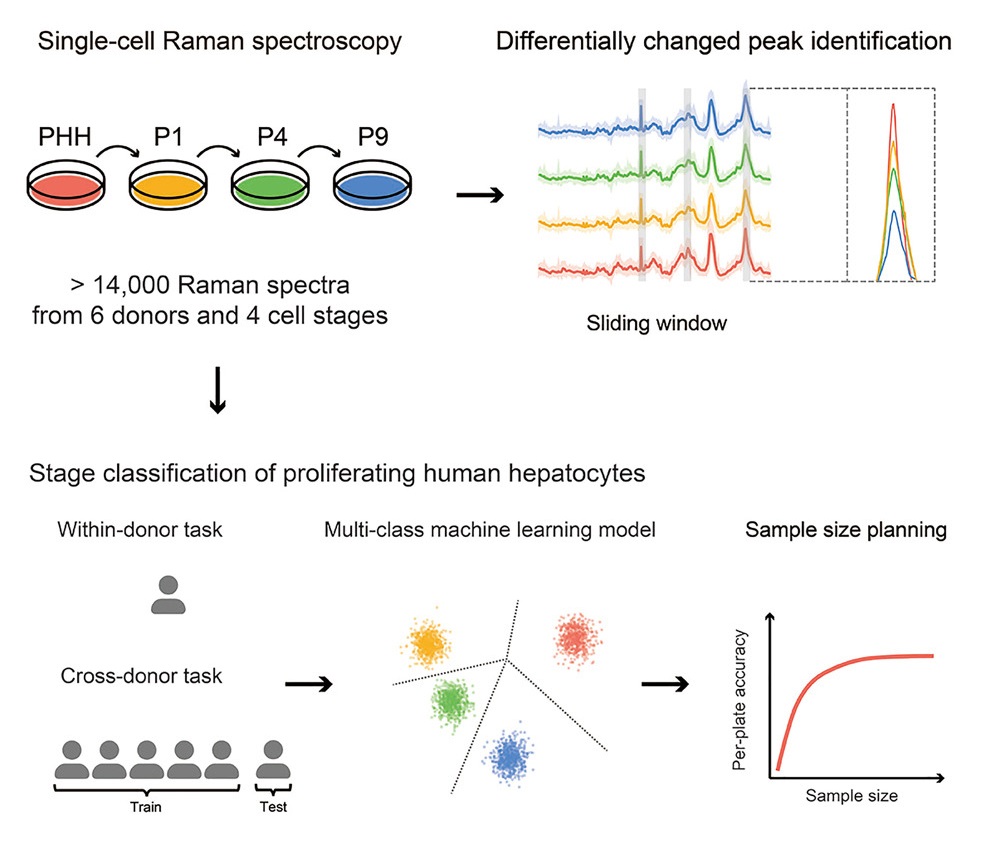
Cell therapy using proliferating human hepatocytes (ProliHHs) is an effective treatment approach for advanced liver diseases. However, rapid and accurate identification of high-quality ProliHHs from different donors is challenging due to individual heterogeneity. Here, we developed a machine learning framework to integrate single-cell Raman spectroscopy from multiple donors and identify different stages of ProliHHs. A repository of more than 14,000 Raman spectra, consisting of primary human hepatocytes (PHHs) and different passages of ProliHHs from six donors, was generated. Using a sliding window algorithm, potential biomarkers distinguishing the different cell stages were identified through differential analysis. Leveraging machine learning models, accurate classification of cell stages was achieved in both within-donor and cross-donor prediction tasks. Furthermore, the study assessed the relationship between donor and cell numbers and its impact on prediction accuracy, facilitating improved quality control design. A similar workflow can also be extended to encompass other cell types.
January
2023
December
- We welcome new graduate students Jingxuan Cai and Jiao Yuan to join our group!
- Congrats to Bihan Shen for being awarded for “2023 Best Poster Award” in GRAD !
November
- Welcome Jingxuan Cai and Jiao Yuan for Lab Rotation!
- Welcome Yongqi Bei, Huicheng Ye and Feifei Sun for internship!
October
- Congrats to the success of the 2023 Bioinformatics and Computational Biology Young Talents Frontier Forum!
- Congrats to professor Li for receiving the 2023 Young Star Award of Shanghai Society for Bioinformatics.
September
- Welcome Qianxi Jia and Xintong Huang for Lab Rotation!
July
- Welcome Fei Guo, Wenwen Zhang and Chenyihang Xiong for summer internship!
- Welcome laboratory manager Zhixuan Tang to join our group!
- Congrats to Bihan Shen and Siyu Jing for being awarded for the 2023 Shanghai Branch of the Chinese Academy of Sciences “Rising Star in Technological Breakthroughs Award” !
- Congrats to professor Li for receiving the Second Outstanding Scientific Research Achievement Award for Female Scientists of the Chinese Biophysical Society of China.
May
- Cancer Systems Biology group contributes a review entitled “Single-cell and spatially resolved transcriptomics for liver biology” in Hepatology.
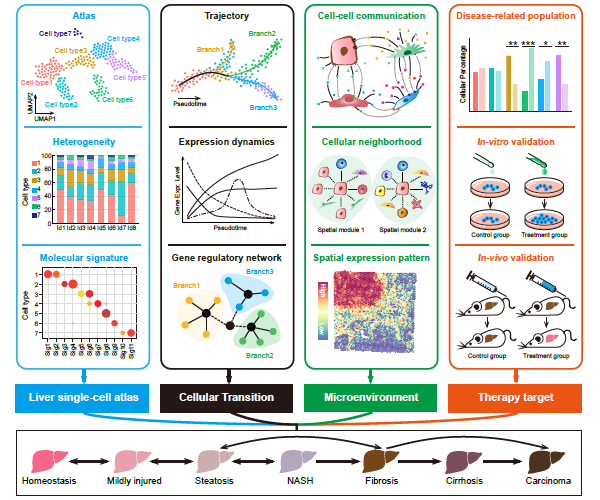
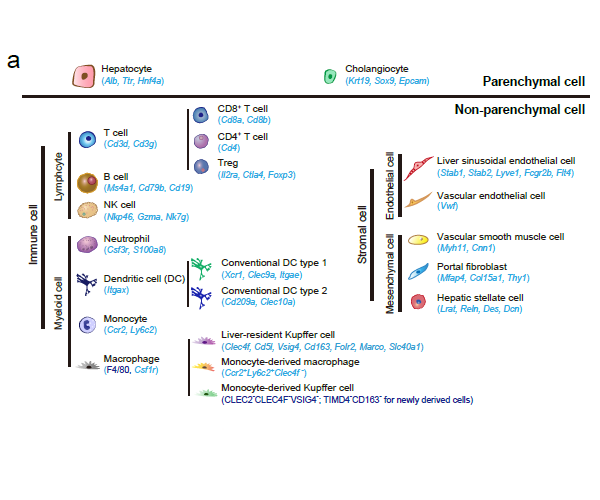
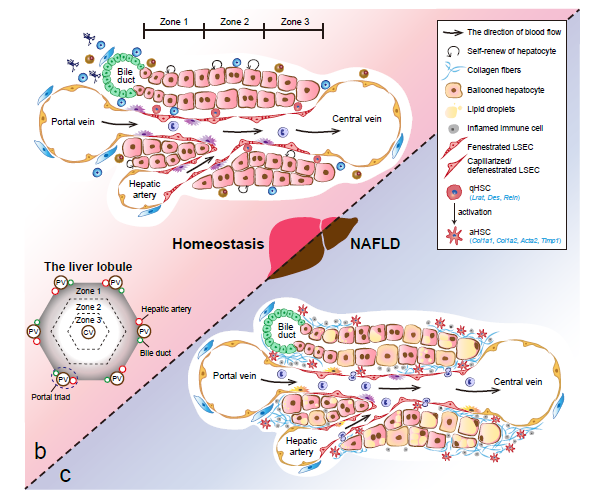
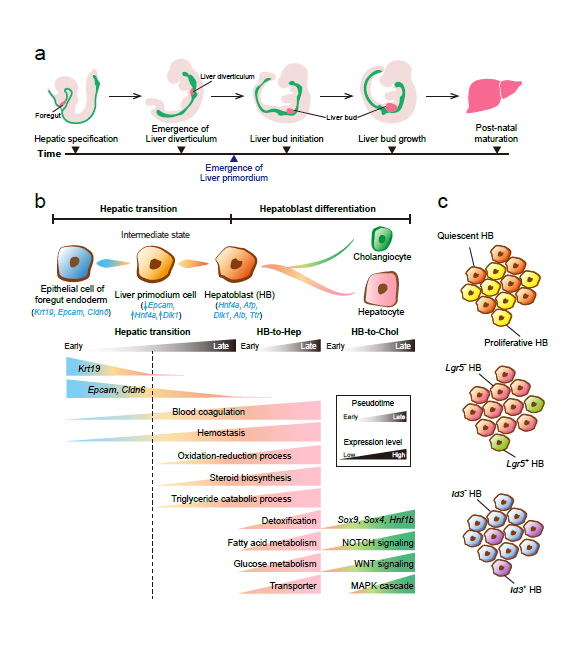
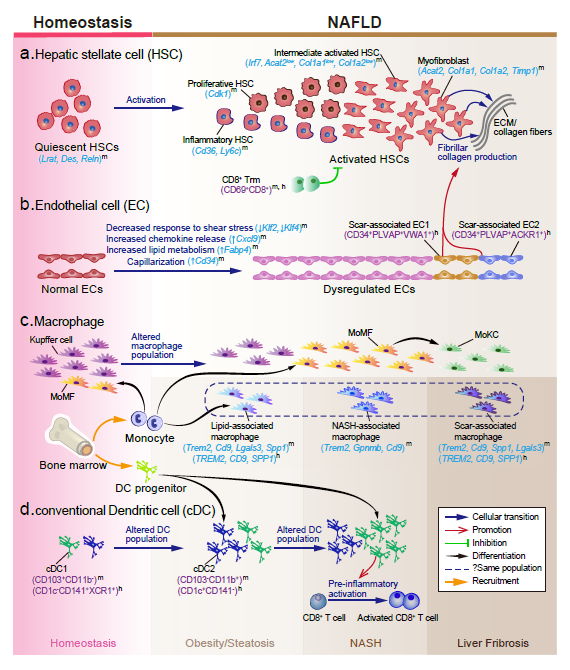
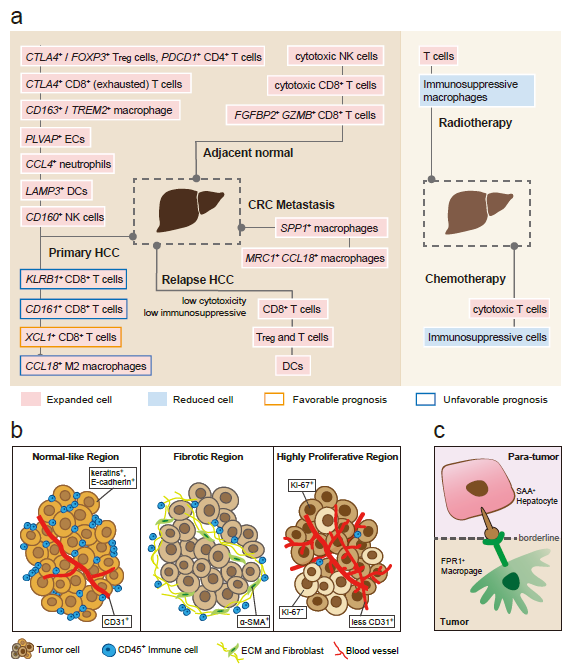
Recently, our lab contributes a review article entitled “Single-cell and spatially resolved transcriptomics for liver biology”. We introduced the basic knowledge about single-cell and spatially resolved techniques and the state-of-art computational tools used for data processing. Four common scenes were summarized when applying these techniques in the study of liver biology: (a) constructing the liver single-cell atlas; (b) the deduction of cellular transitions; (c) the characterization of tissue microenvironments; (d) the identification of potential therapy targets (Fig. 1). The four scenarios were seen in the settings of liver homeostasis, embryonic liver development, liver regeneration, non-alcoholic liver disease (NAFLD) and liver cancer. In each topic, we presented the cutting-edge progresses uncovered by single-cell and/or spatial techniques, such as the single-cell atlas of the liver under both diseased and homeostatic conditions (Fig. 2a), transcriptomic zonal patterns of hepatic cells (Fig. 2b), developmental trajectory of embryonic liver (Fig. 3), cell identity conversion of parenchymal cells upon injury, NAFLD-associated cell subtypes (Fig. 4), and cancer-associated cell populations within tumor microenvironments (Fig. 5). We highlighted the computational strategies that help achieve these accomplishments; and discussed the outstanding questions remaining elusive. Lastly, we pointed out the challenges when applying these technologies to the liver biology, and for each of which, we proposed possible solutions.
- Welcome Jingxuan Cai for summer internship!
April
- Congrats to professor Li for receiving the 2023 China Cell Biology Society-TianNeng Life Science Young Female Scientist Career Development Fund, category B !
February
- Cancer Systems Biology group contributes a research paper entitled “Kupffer cell-derived IL-6 is repurposed for hepatocyte dedifferentiation via activating progenitor genes from injury-specific enhancers” in Cell Stem Cell.
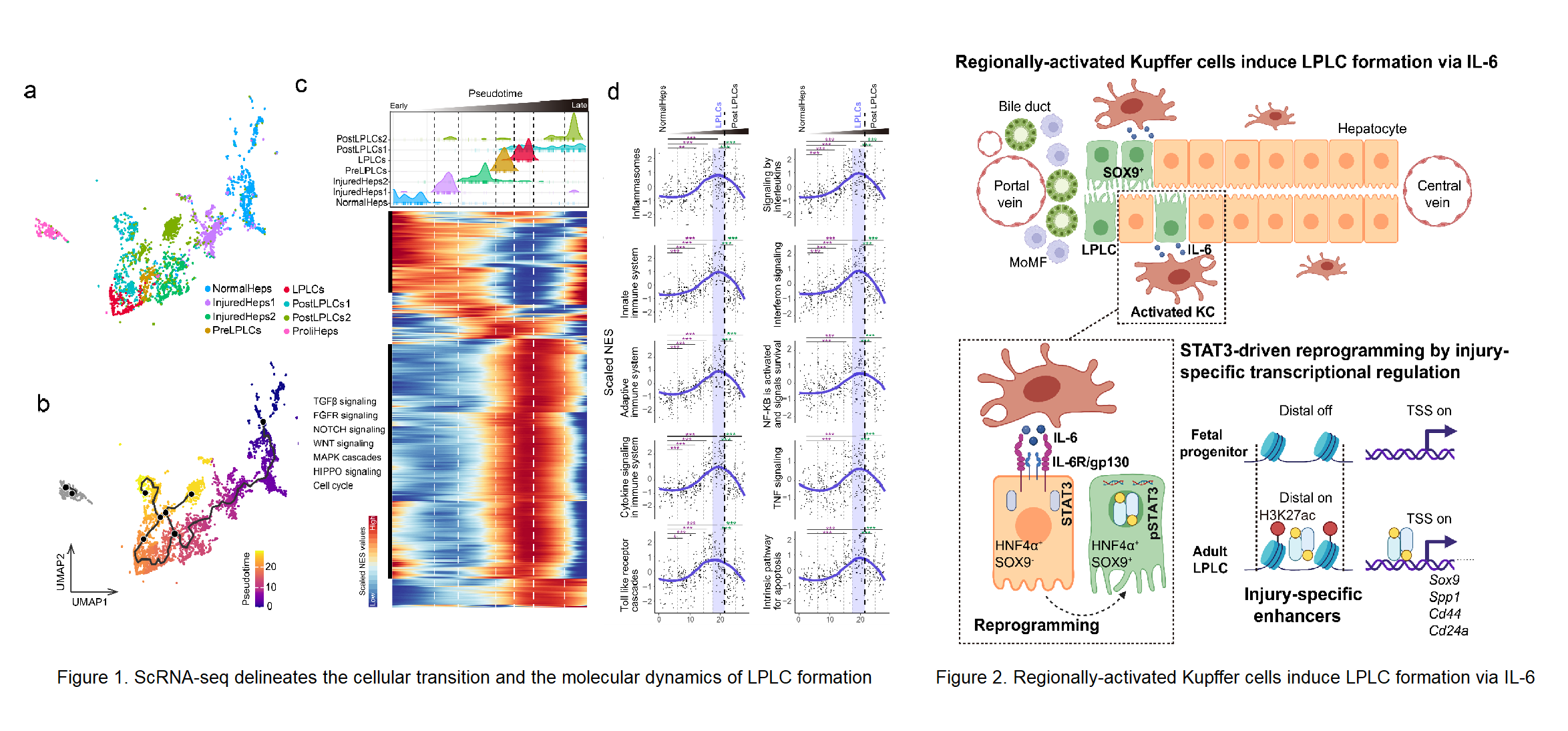
Stem cell-independent reprogramming of differentiated cells has recently been identified as an important paradigm for repairing injured tissues. Following periportal injury, mature hepatocytes re-activate reprogramming/progenitor-related genes (RRGs) and dedifferentiate into liver progenitor-like cells (LPLCs) in both mice and humans, which contribute remarkably to regeneration. However, it remains unknown which and how external factors trigger hepatocyte reprogramming. Here, by employing single-cell transcriptional profiling we reconstructed the cellular trajectory of LPLC formation and deciphered the key pathways underlying cellular state transition during the process (Figure 1). We found that immune related pathways were highly correlated with LPLC formation. By utilizing lineage-specific deletion tools, we uncovered that periportal-specific LPLC formation was initiated by regionally activated Kupffer cells but not peripheral monocyte-derived macrophages (Figure 2). Unexpectedly, using in vivo screening, the proinflammatory factor IL-6 was identified as the niche signal repurposed for RRG induction via STAT3 activation, which drove RRG expression through binding to their pre-accessible enhancers. Notably, RRGs were activated through injury-specific rather than liver embryogenesis-related enhancers. Collectively, these findings depict an injury-specific niche signal and the inflammation-mediated transcription in driving the conversion of hepatocytes into a progenitor phenotype (Figure 2). Lu Li, Lei Cui from Shanghai Institute of Biochemistry and Cell Biology (SIBCB), Center for Excellence in Molecular Cell Science, CAS and Ping Lin from Cancer Systems Biology group at Shanghai Institute of Nutrition and Health (SINH), CAS serve as co-first authors. Professor Lijian Hui from SIBCB, Center for Excellence in Molecular Cell Science, CAS, Professor Hong Li from Cancer Systems Biology group at SINH, CAS and Professor Yixue Li from SINH, CAS serves as the co-corresponding authors. This work was supported by the funding from Chinese Academy of Sciences, the National Key Research and Development Project, the National Natural Science Foundation of China, CAS Youth Innovation Promotion Association, Shanghai Science and Technology Committee, Shanghai Municipal Science and Technology Major Project, and the Shanghai Sailing Program.
For more information, please refer to: BioArt iNature
- Congrats to Siyu Jing for being awarded for “2022 Best Research Report Award” in GRAD !

2022
December
-
Congrats to professor Li for being awarded for “2022 Excellent Member Award, Youth Innovation Promotion Association, Chinese Academy of Sciences” !
-
Cancer Systems Biology group contributes a benchmarking paper entitled “A systematic assessment of deep learning methods for drug response prediction: from in vitro to clinical applications” in Briefings in Bioinformatics.
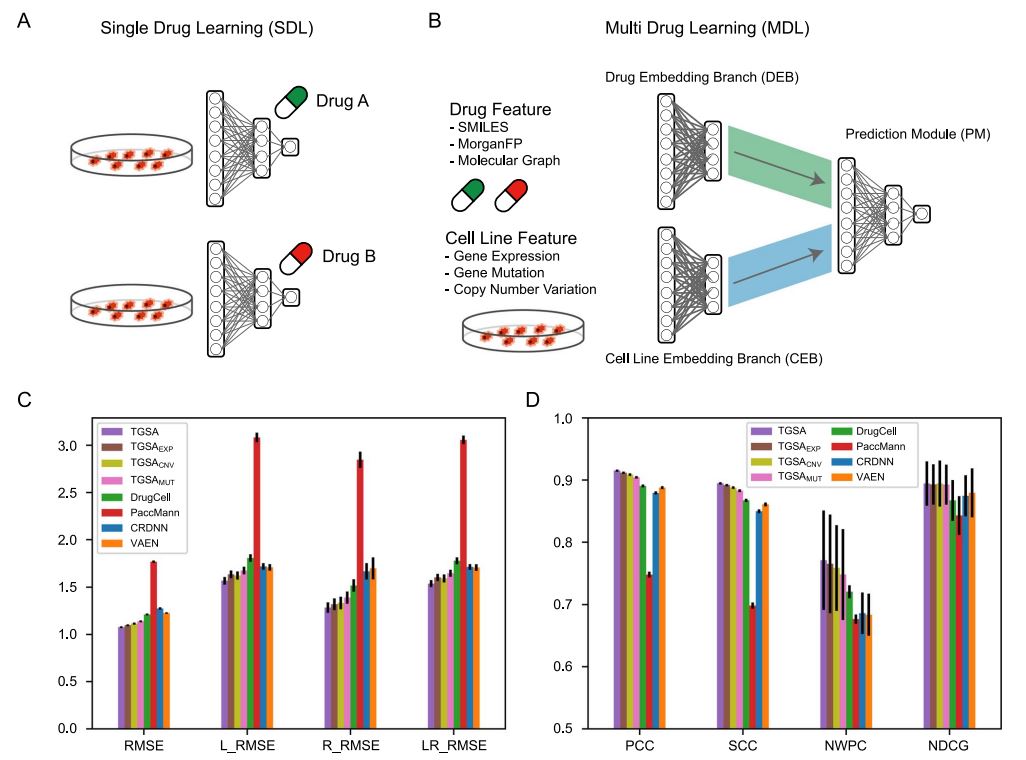
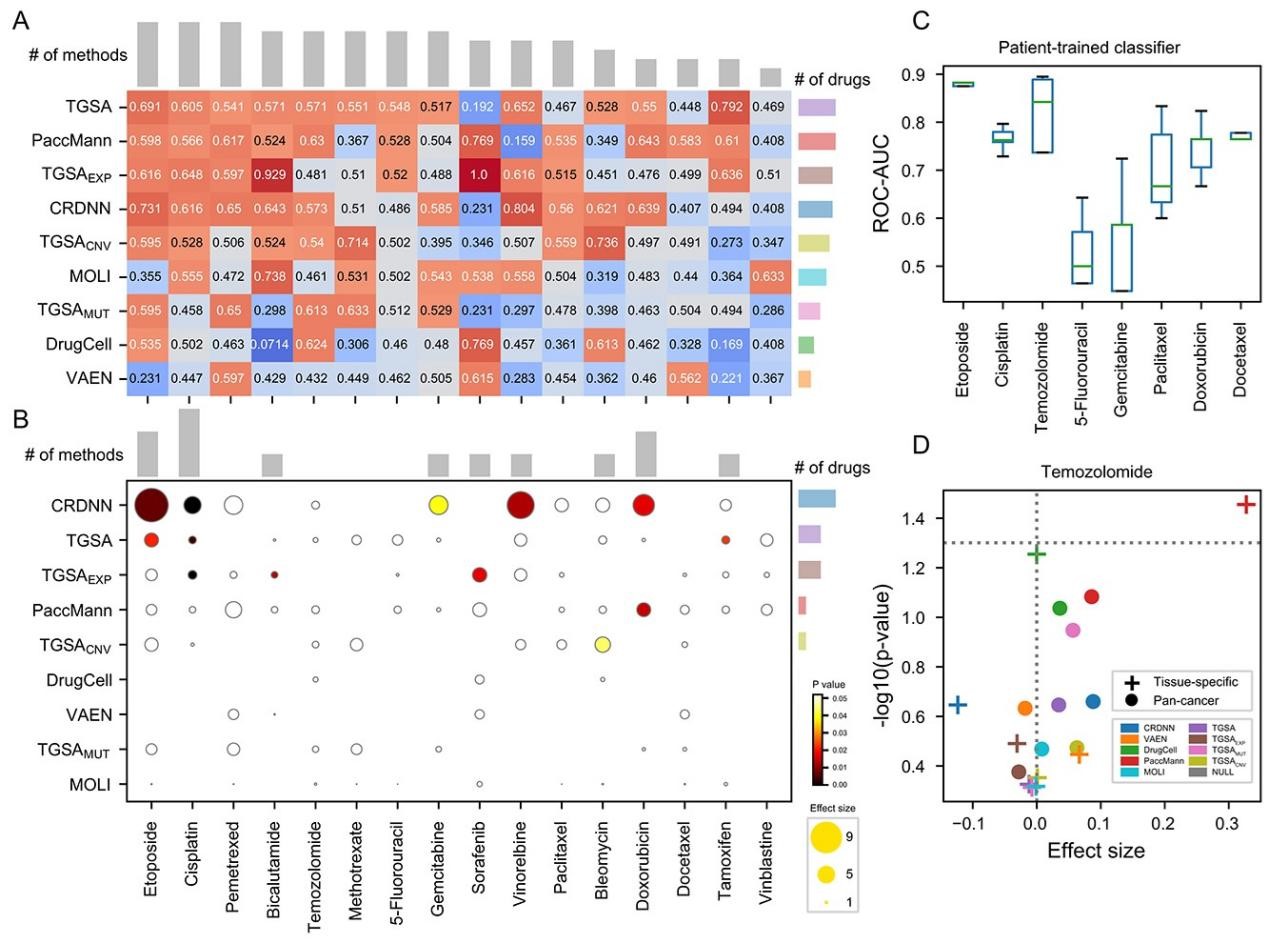
Drug sensitivity prediction is an important and challenging problem in both bioinformatics and translational medicine. Various categories of methods have been developed and compared. However, previous benchmark studies have rarely discussed the predictive ability of preclinical models on clinical patients, and not comprehensively covered the paradigms of deep learning methods. To provide a more rigorous assessment, the performance of six representative deep learning methods for drug response prediction in multiple application scenarios was benchmarked, including the overall prediction accuracyusing nine evaluation metrics, predictability of each drug, potential associated factors and transferability to clinical cohorts. Most methods show promising prediction within cell line datasets (Figure 1). TGSA, with its lower time cost and better performance, is recommended. The performance metrics decrease when applying models trained on cell lines to patients (Figure 2), due to the differences between cell lines and patients, the lack of drug sensitivity related features and the reduction of statistical power due to small sample sizes. However, clinical response on some drugs can still be distinguished using CRDNN and TGSA models. These assessments provide a guidance for researchers to choose appropriate methods, as well as insights into future directions for the development of more effective methods in clinical scenarios. Bihan Shen and Fangyoumin Feng serve as co-first authors. Professor Hong Li serves as the corresponding author. Kunshi Li and Ping Lin also contributed to this work. We thank Bio-Med Big Data Center at Shanghai Institute of Nutrition and Health, Xin Li (Shanghai Institute of Nutrition and Health, CAS) and Wenju Cai (Guizhou science data center Gui’an Supercomputing Center) for providing computational resource and discussions. This work is supported by National Natural Science Foundation of China, Natural Science Foundation of Shanghai, National Key R&D Program of China, CAS Youth Innovation Promotion Association and the development fund for Shanghai talents.
- We welcome new graduate students Wanhong Chen, Xufeng Chen and Xueliang Li to join our group!
November
- Welcome Xufeng Chen for Lab Rotation!
August
- Welcome Wanhong Chen, Xueliang Li, Zhaozhen Du for Lab Rotation!
July
- The Cancer Systems Biology (CSB) group is recruiting bioinformatics graduate students, postdocs, and research scientists. Please contact us by email (lihong01
 sinh.ac.cn).
sinh.ac.cn).
- Congrats to professor Li for being awarded for the 2021 Shanghai Science and Technology System “Youth May Fourth Medal” !
- Congrats to Dr. Feng for the completion of Postdoctoral Fellowships!